Abstract
Primary central nervous system lymphoma (PCNSL) is an infrequent and aggressive non-Hodgkin lymphoma usually classified as diffuse large B-cell lymphoma (DLBCL), being most of the cases more closely related to activated B-cell type (ABC). PCNSL is associated with dismal prognosis, due in part to the difficulty for many chemotherapeuticals to cross the blood brain barrier.
Selinexor (KPT-330) is a Selective Inhibitor of Nuclear Export (SINE) compound that binds to and inactivates XPO-1 protein and induces anti-tumor effects mainly due to forced nuclear retention and activation of tumor suppressor proteins. Selinexor, that has shown excellent brain penetrance and promising results in pre-clinical models of glioblastoma, can inhibit both BCR and NF-kB signaling in malignant B-cells.
In order to provide a pre-clinical rationale for the design of new therapeutic strategies in the PCNSL, we assessed the role of XPO-1 inhibition using intracerebral xenograft murine models.
To compare the sensitivity of DLBCL-derived cell lines to selinexor, we determined the inhibitory concentration 50 (IC50) in terms of survival and proliferation after 96 hours in 4 ABC and 5 GCB DLBCL cell lines. Analysis showed that DLBCL cell lines have equivalent sensitivity to selinexor, regardless the cell of origin. Since SINE compounds have been shown to inhibit BCR signaling, we next tested the potential synergy between ibrutinib and selinexor. In 3 out of 4 ABC-DLBCL cell lines there was a strong synergy. In contrast, none of the 3 GCB-DLBCL cell lines analyzed were sensitive to up to 100 µM single agent ibrutinib; interestingly, however, treatment with selinexor sensitized SUDHL4 (GCB) cells to ibrutinib and the combination index values indicated strong synergism between the two drugs.
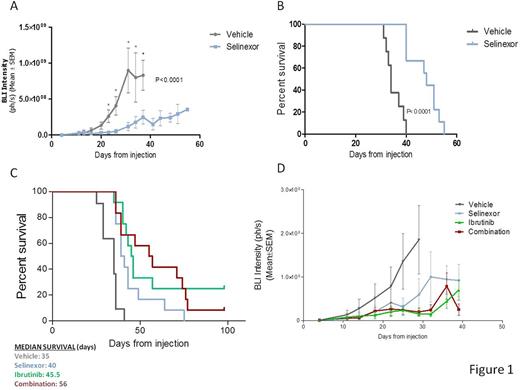
We then established an orthotopic xenograft model of PCNSL by stereotactic injection of OCI-Ly10 cells stably transfected with luciferase into the brain parenchyma of nude athymic mice. Eleven days after the injection of cells all animals had developed detectable tumors confined to the CNS. Tumor size was measured and animals were randomly distributed into drug or vehicle experimental group. At this time point mice were treated with 5mg/kg of selinexor or vehicle via oral gavage three times a week; subsequently, bioluminiscence was assessed twice a week. Treatment with selinexor significantly blocked tumoral growth (two-way ANOVA: p< 0.0001; figure 1A). Specific time-point analysis showed that differences were significant as soon as 12 days after treatment (2,255e+008 +/- 8,648e+007 vs 8,670e+006 +/- 1,981e+007). Also, treatment with selinexor increased mice survival, with a median survival of 48 days in the treatment group compared to 34 days in the vehicle group (p< 0.0001; figure 1B). At final point, histopathological analysis showed diffuse infiltration in meninges and cerebral parenquima of highly proliferative CD20-positive B-cells (virtually 100% Ki-67-positive malignant B cells). Since the infiltration of tumoral associated macrophages (TAMs) has been related to worse prognosis in different lymphomas, including PCNSL, we analyzed the infiltration by F4/80+ TAMs after two weeks of treatment with selinexor or vehicle. We observed that all tumors from vehicle-treated mice were infiltrated with TAMs, whereas treatment with selinexor significantly reduced the presence of TAMs as assessed by immunohistochemistry, especially in the parenquima. Next, we evaluated the potential synergy between ibrutinib and selinexor in vivo. For that 12 mice with equivalent tumor size were assigned to each of the following four groups: selinexor only (5mg/kg twice a week), ibrutinib only (25mg/kg daily), combination or vehicle. The combination of both drugs significantly increased survival compared to both treatments as single agent, whereas there was no significant difference between ibrutinib and selinexor treatments alone (figure 1C). Tumoral growth was equally blocked by all treatments (figure 1D). Currently, we are evaluating the effect of the combination in the infiltration and immunophenotype of TAMs, including M1 vs M2 phenotype and PD1 expression. Results will be available at the time of the meeting.
Selinexor proved to be an active agent in blocking tumoral growth in DLBCL infiltrating CNS, this providing pre-clinical evidence for the development of selinexor as new therapeutic option for PCNSL or DLBCL with CNS involvement.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal